Life Science Factory @ Festival der Zukunft
Improving the world – but how? We partnered up with the Festival der Zukunft for the third time to find out how we can shape our future with technology, science and creativity.
We’re excited to dive into transformative topics in life sciences and entrepreneurship. Join us if you want to explore:
Friday // 13:45 // Theater Stage
Panel: “Can Artificial Intelligence Finally Cure Cancer?” – featuring our community members Christina Port, 2NA Fish and Daniel Soric, Deep LS
Friday // 14:30 // orange room
Life Science Meet-up: Connect and learn within a creative networking space where innovation thrives and ideas come to life – hosted by our Venture Team Martin Strehle, Irina Reimer and Max Zinowsky
Friday // 16:00 // orange room
about the life science factory
vision.
facilities.
programs.
benefits.
Benefits for investors

- Mitigate Risks and Enhance Outcomes: By integrating your portfolio start-ups into the Life Science Factory, you leverage our first-class infrastructure and expertise, significantly enhancing the probability of their success.
- Access to Expert Guidance: Start-ups benefit from continuous support from our specialists, particularly through the critical stages of fundraising and business development, which can improve their stability and growth potential.
- Quality Assurance in Investments: Through our meticulous screening and selection process, we ensure that only the most promising and qualified start-ups are presented to investors, thereby minimizing investment risks.
- Early Investment Opportunities: We provide exclusive early-stage investment opportunities, allowing you to invest in promising start-ups before they catch the broader market’s attention.
- Reliability and Credibility: As an affiliate of Sartorius AG, we stand as a trustworthy partner, ensuring a stable and reliable collaboration for both investors and start-ups.
Benefits for start-ups
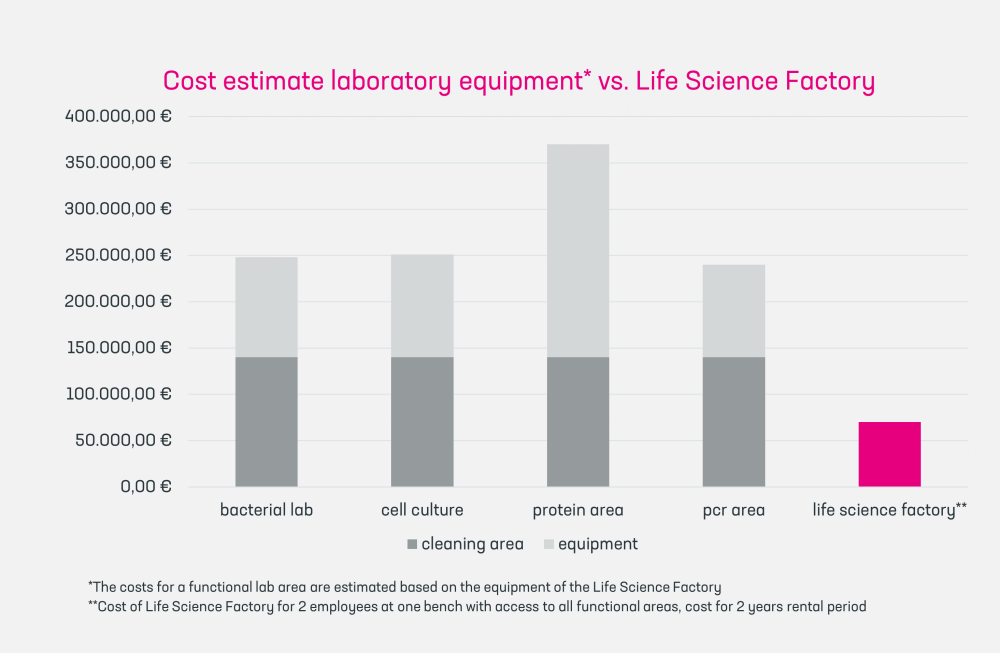
- Advanced R&D Capability: We provide state-of-the-art lab equipment with supervision, allowing start-ups to focus on innovation without the overhead of equipment management.
- Branding and Networking: Through collaboration, we enhance the reputation and visibility of start-ups, expanding their network and opening up new opportunities.
- Operational Flexibility: Our shared lab benches and flexible coworking spaces offer a quick start and scalable options to accommodate the changing needs of start-ups.
- Regulatory and Pre-operational Support: We support start-ups with essential services before the rental period begins, including communication with authorities, easing the regulatory setup process.
- Knowledge Exchange and Skill Development: Our interdisciplinary teams and alumni network deepen know-how and facilitate exchange, enriching the life science community’s collective expertise.
- Long-term Growth and Connections: We connect founders with relevant experts and partners, and provide referrals to accelerator programs, ensuring sustained growth and benefits from our network.
- Educational Opportunities and Community Engagement: Start-ups benefit from free participation in our programs and events, promoting continuous learning and engagement within the community.
discover the life science factory
Explore our facilities in Göttingen with our virtual tour. Step into our innovative spaces designed for collaboration and progress, and explore all we have to offer.
Discover our new facilities developed in collaboration with Helmholtz Munich in Munich Neuherberg. This innovative hub provides start-ups with laboratory spaces and access to extensive support services.
Lets Connect
Contact Us
Jul
Deep Dive: Innovative Teams with Elisabeth Karg
July 16, 2025 / 4:00 pm — 5:30 pm
In this session, we'll dive into the dynamics of team formation in life science start-ups, focusing on the crucial role of the founders. We'll explore strategies for building a cohesive team, emphasizing the importance of diversity, interdisciplinary collaboration, and fostering a culture of continuous learning. These elements are key to transforming your start-up into a thriving and innovative enterprise
Sep
ELSA Bootcamp
September 04, 2025 / 9:00 am
On September 4, 2025, ELSA kicks off with an intensive opening day. Teams undergo a stress test, conduct needs assessments with lead coaches, and conclude the day with a special networking event.
Oct
ELSA 1st Check-in
October 15, 2025 — October 16, 2025 / 8:45 am
On October 15-16, 2025, the first check-in of the ELSA series takes place. Teams showcase their progress, receive individual feedback, and refine their projects in focused coaching sessions.
Nov
life science factory @ Bio-Europe
November 03, 2025 — November 05, 2025 / 9:00 am
BIO-Europe is one of the most significant international events for the biotechnology and pharmaceutical industries. The event connects stakeholders from academia, industry, and investment, offering a platform to explore the latest trends and innovations.
Nov
ELSA 2nd Check-in
November 12, 2025 — November 13, 2025 / 8:45 am
On November 12-13, 2025, the second check-in of the ELSA series will take place. Teams will engage in hands-on workshops and personal coaching to refine their strategies and set new directions for their work.
By displaying this Google map you agree to Google’s use of cookies. This may include analytics, personalization and ads.
Learn more



